Quality Control & Certificates
Nutritech is a fully licensed contract manufacturing with the following memberships and certificates to ensure we provide the best service and maintain in high standard of the natural products industry to our clients.
Built and run with pharma grade operation standards, Nutritech adheres and surpasses Health Canada GMP and US FDA standards for manufacturing OTC natural health care supplements.
Nutritech’s QC/QA team consists of pharmaceutical scientists and chemists, well versed in their fields with several decades of experience between them. Our GMP facilities are audited and validated by 3rd party experts.
• Nutritech GMP facilities
• Certificates & compliance
• Regulatory services
• Analytical Laboratory Testing







Certificates & Compliance
1. GMP /Site License
2. Natural Product Number (NPN) License
3. International Trade Certificate ( ITC)
4. US FDA food fertilities Registration
5. Vegetarian Certificate
6. Halal certificate
7. BSE Certificate
8. Member of CACDS
9. Member of CHFA
VIEW CERTIFICATE
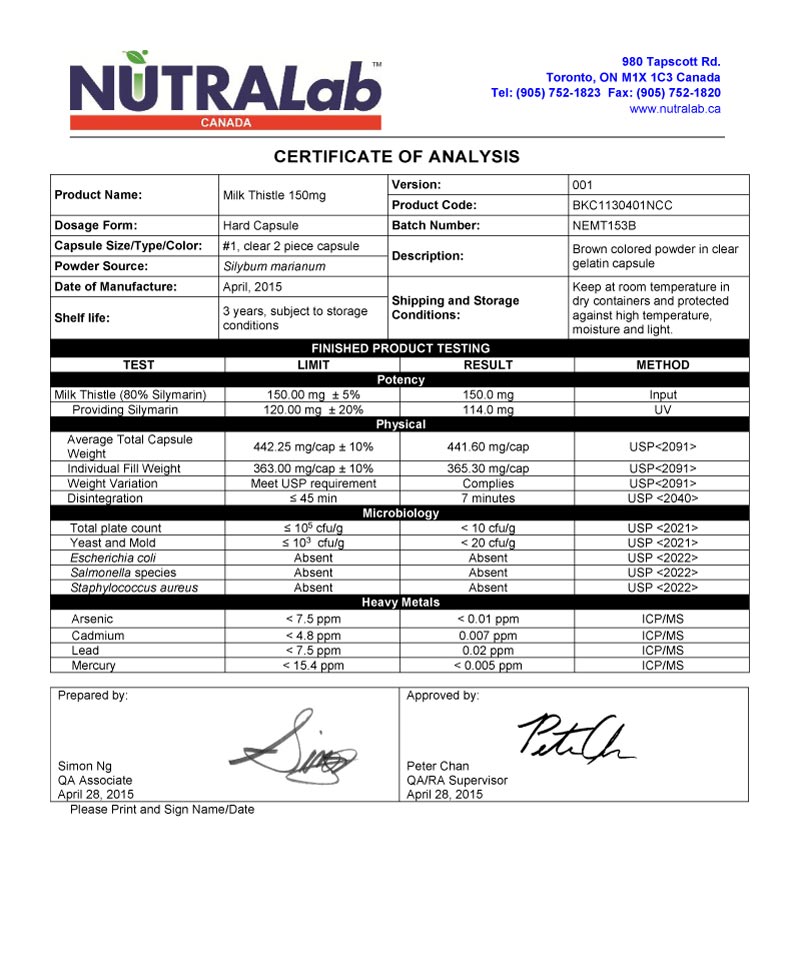
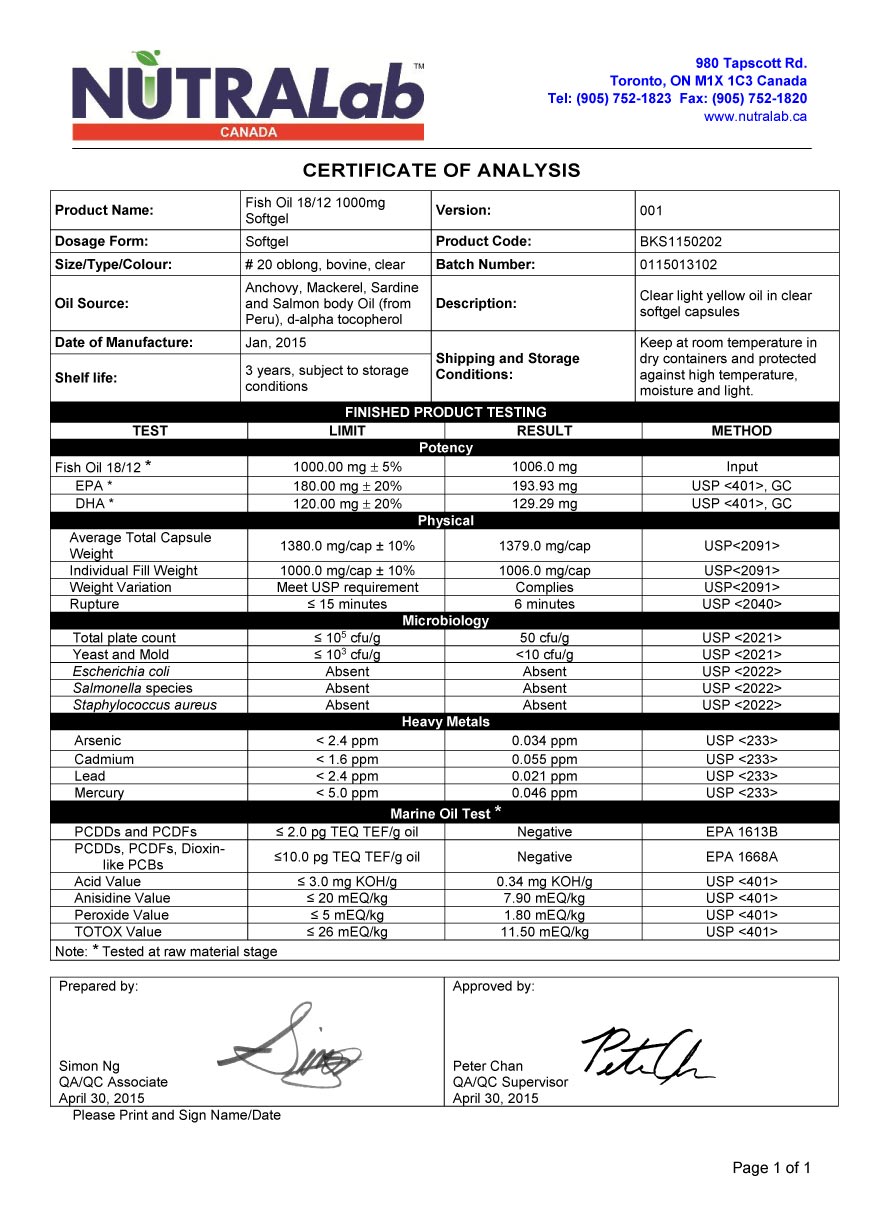
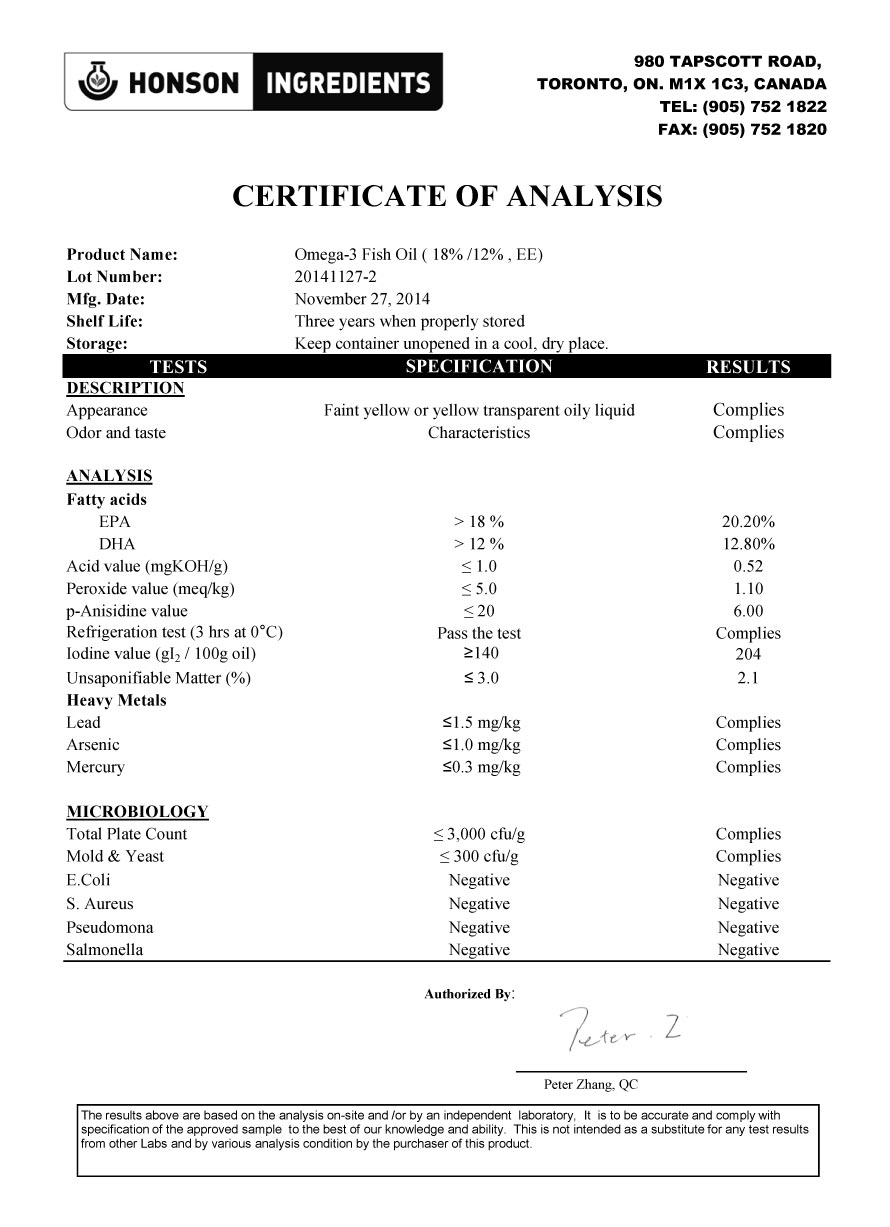
OF ANALYSIS
Nutritech is a fully licensed contract manufacturing with the following memberships and certificates to ensure we provide the best service and maintain in high standard of the natural products industry to our clients:
GMP/Site License
Good Manufacturing Practice (GMP) is a term that is recognized worldwide for the control and management of manufacturing and quality control testing of foods, pharmaceutical products, and medical devices.
The GMPs are designed to be outcome based, ensuring safe and high quality products, while giving manufacturers, packagers, labellers, importers and distributors of Natural Health Product’s (NHP’s) the flexibility to implement quality systems appropriate for their product lines and businesses.
Nature Product Number (NPN) license
All natural health products require a product license before they can be sold in Canada. Obtaining a license will require submitting detailed information on the product to Health Canada, including: medicinal ingredients, source, potency, non-medicinal ingredients and recommended use. Once a product has been assessed and granted market authorization by Health Canada, the product label will bear an eight digit product license number preceded by the distinct letters NPN, or, in the case of a homeopathic medicine, by the letters DIN-HM. This number on the label will inform consumers that the product has been reviewed and approved by Health Canada for safety and efficacy.
International Trade Certificates (ITCs):
ITC’s are documents that contain information about a product’s or site’s regulatory status in Canada which serve to assure foreign regulatory authorities that NHPs exported to their countries can be marketed in Canada and meet the NHPR.
ITC’s to be issued for natural health products that have a valid natural product number (NPN) which indicates that the Natural Health Product Directorate of Health Canada has reviewed the product licence application and has deemed it safe and effective. eported to their countries can be marketed in Canada and meet the NHPR.
U.S. FDA food facility registration
US FDA is required for all companies that manufacture, process, pack, or store food, beverages, or dietary supplements that may be consumed in the United States. to be permitted to inspect the facility at the times and in the manner permitted by the FD& C Act.
Vegetarian ( capsule) certificate
Nutritech offers vegetable capsules that are suitable for vegans taking dietary supplements.
Halal Certificate
We manufacture natural supplements that are halal certified, which means that our products are permissible under Islamic law.
BSE Certificate
Our BSE certificate certifies that the gelatin capsules we use are free of Bovine Spongiform Encephalopathy (BSE). Our capsules also meet all the requirements of current European Pharmacopoeia (EP) and the United States Pharmacopoeia (SP).
Member of CACDS
As members of The Canadian Association of Chain Drug Stores (CACDS) we represent supply categories and services in the retail pharmacy industry, such as pharmaceuticals, health and wellness products, self-care medications, and other consumer products.
CACDS is there to ensure a strong chain drug store sector which provides Canadian consumers with access to high quality products and health care services.
Member of CHFA
The Canadian Health Food Association (CHFA) is Canada’s largest trade association dedicated to natural health and organic products. CHFA represent their member of operating in a good standards of natural health industry in Canada.
CHFA members include manufacturers, retailers, wholesalers, distributors, and importers of natural and organic products. These can include foods, vitamin and mineral supplements, herbal products, homeopathics, sports nutrition products, health and beauty aids and more.






PRODUCTS
© 2016-2017 Nutritech Asia Group LTD. All rights reserved.